Our pipeline
Purposeful science. Lasting positive impact.
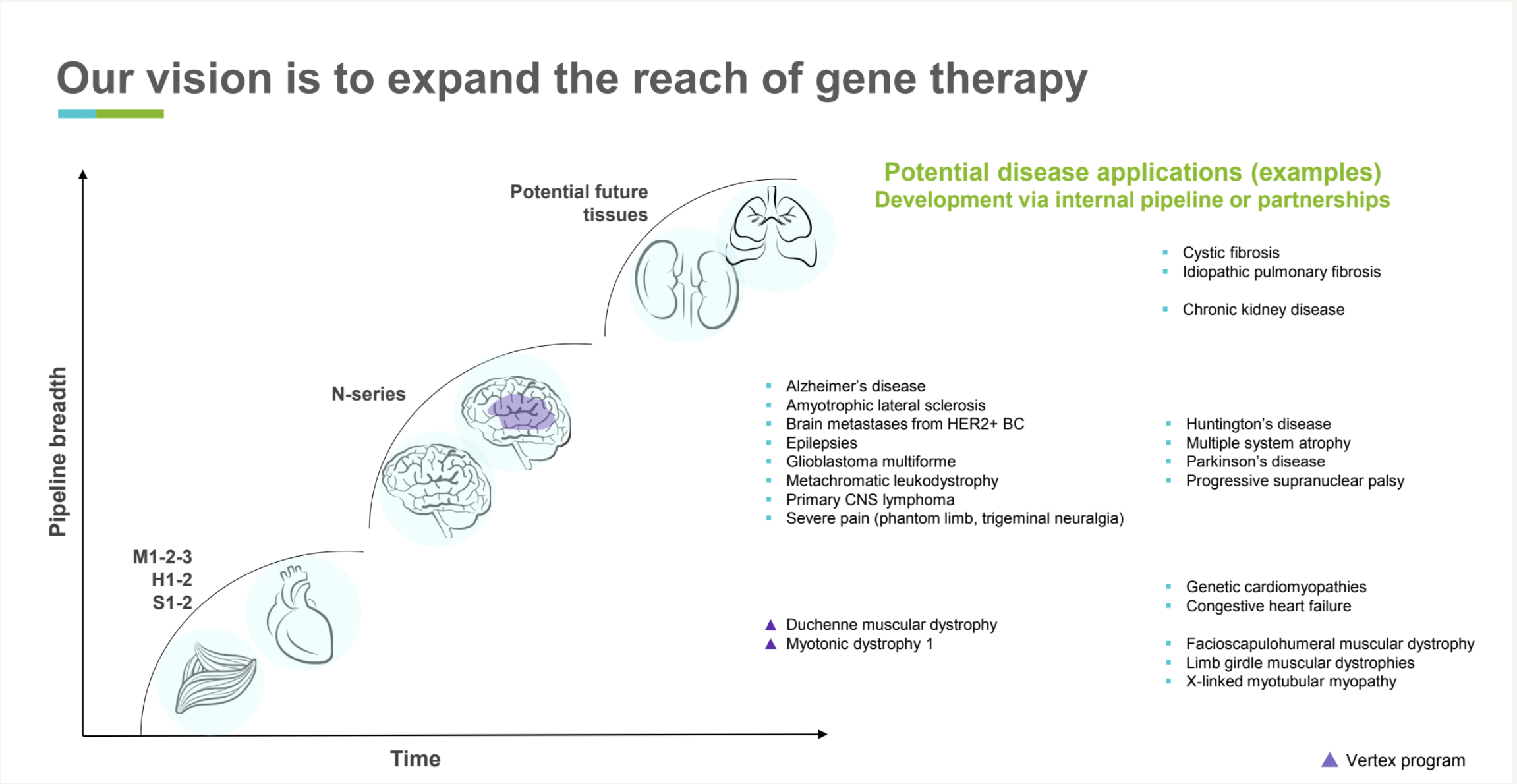
We are building a deep and expansive pipeline of rationally designed gene therapy product candidates, pioneering a shift to treat not only rare but also prevalent diseases. We apply our ART platform to develop first-in-class or best-in-class product candidates using our proprietary capsids, promoters, and manufacturing approaches. We typically design multiple capsids and gene constructs with one as the lead and others as backups. We treat manufacturability as a critical dimension, similar to efficacy and safety, aiming to define and control an initial manufacturing process that we can carry through the development path of the product candidate.
Our current internal pipeline focus is to develop gene therapies that treat nervous system, cardiac, and muscle diseases. In addition to establishing our own pipeline, we plan to benefit patients through strategic partnerships. For example, through a collaboration with Vertex Pharmaceuticals, Affinia Therapeutics is combining our capsid design expertise with Vertex’s scientific, clinical, and regulatory capabilities to accelerate the development of genetic medicines for people affected by Duchenne muscular dystrophy (DMD), myotonic dystrophy type 1 (DM1), and cystic fibrosis (CF).
References
- Rosenberg GA. Neurological diseases in relation to the blood brain barrier. J. Cereb Blood Flow Metab. 2012;32:1139-1151.
- Metachromatic Leukodystrophy. National Organization for Rare Disorders (NORD). Accessed 18 Jun 21. https://rarediseases.org/rare-diseases/metachromatic-leukodystrophy/.
- Stanek LM, Calcedo R, Mastis B, et al. CSF delivery of Anc80L65 in the non-human primate brain results in widespread gene transfer throughout the central nervous system compared to AAV9. Digital poster presented at: 2021 American Society of Cell and Gene Therapy; May 11-14; virtual.
- Cancer Stat Facts: Female Breast Cancer. National Cancer Institute (NCI). Accessed 29 Aug 21. https://seer.cancer.gov/statfacts/html/breast-subtypes.html
- Zimmer AS, Van Swearingen AED, Anders CK. HER2-positive breast cancer brain metastasis: a new and exciting landscape. Cancer Rep. 2020 Sep 3;e1274.